Injection Augmentation of the Vocal Folds with Calcium Hydroxylapatite

Figure 1.
Endoscopic visualization of the vocal folds during surgery. a) Bowed, atrophic vocal folds. c) Medialized, plump vocal folds after injection of 0.15cc of CaHA bilaterally.
Synthetic calcium hydroxylapatite (Radiesse, BioForm Inc., Franksville, WI, USA) was approved for vocal fold augmentation by the United States Food and Drug Administration Center for Devices and Radiological Health in January, 2002. Calcium hydroxylapatite (CaHA) is the primary mineral constituent of bone and teeth. It is highly biocompatible (well accepted for implantation by the body) and has the potential for long-term and even permanent vocal fold augmentation. We have been using the implant for injection into the vocal fold for slightly over 2 years. We are therefore unable to state with certainty that the implant is permanent. The majority of patients that we have injected, however, have had long-term improvement. There is some resorption of the CaHA carrier at three months. We therefore tell all of our patients that a second injection may be necessary 3-4 months after the initial procedure. Our re-injection rate, however, is only 15%. We have injected over 100 vocal folds with excellent results. The implant can be injected into a paralyzed (immobile) vocal fold or a paretic (weak but mobile) vocal fold. We also frequently utilize the implant to reverse age-related changes in the vocal fold (presbylarynx) – the so-called Voice Lift Procedure. Patients with Parkinson’s hypophonia have also benefited from the procedure. We routinely inject both vocal folds at the same time.
Injection augmentation of the vocal folds with CaHA is best reserved for small and medium sized glottal gaps (space between the vocal folds). Larger gaps may be better treated with an implant placed externally through a small cervical incision (medialization laryngoplasty +/- arytenoid repositioning).
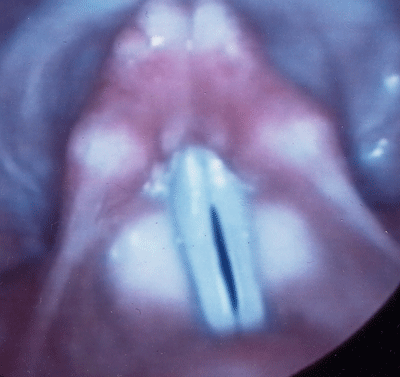
Figure 2.
Videostroboscopic image of bowed, slightly atrophic vocal folds with a 2mm gap at the point of vocal process contact. This patient would be a perfect CaHA injection patient.
CaHA can be injected into the vocal folds percutaneously (through the skin) or per-orally (through the mouth). The procedure can be performed in a patient who is awake, or in an individual who is asleep under the effects of general anesthesia. Because the implant is potentially permanent and the implant can have detrimental effects on vocal fold vibration if injected superficially, we perform all of our injections per-orally under anesthesia with microscopic guidance. This ensures highly accurate injections.
The patients’ eyes and teeth are protected and microlaryngoscopy is performed. We use a Hunsaker Mono-Jet subglottic ventilation tube (Xomed Surgical Products; Jacksonville, Fla.). Use of the Hunsaker tube affords an unobstructed view of both vocal folds while providing continuous ventilation. We use a Brünings-type laryngeal injector that allows for precise implant delivery (Karl Storz, Tuttlingen, Germany). Each click of the Storz injector delivers 0.04cc of implant. This helps prevent over-injection. A syringe is packaged with the product. If the Storz injectior is not available, the syringe that the implant comes packaged in is adequate.
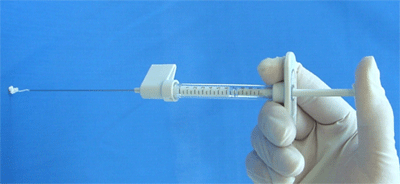
Figure 3.
Synthetic calcium hydroxylapatite with pre-packaged injection syringe. 25G needle for per-oral laryngeal injection is not shown.
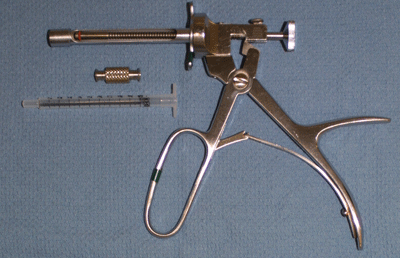
Figure 4.
Storz injection syringe. Each click delivers 0.04cc of implant.
The location of CaHA injection is crucial. The implant must be delivered deep to the thyroarytenoid (TA) muscle. A superficial injection could severely compromise mucosal vibration. Placing a hand on the neck from the outside and pushing the thyroid cartilage medially toward the tip of the needle assists with injection deep to TA muscle right up against the inner perichondrium of the thyroid cartilage. The anterior aspect of the vocal process is a useful landmark. We inject just anterior and lateral to the vocal process. The amount of CaHA to inject depends on the degree of vocal fold mobility, the need for a bilateral injection, the degree of atrophy, and the position of the vocal fold if immobile. The gel carrier constitutes approximately 30% of the implant by volume and will eventually be absorbed. We have noticed some absorption of the gel carrier in some individuals at 3 months post-implantation. We take this 30% absorption into consideration when injecting the CaHA. Because the individual is under general anesthesia and the surgeon is unable to titrate the amount of CaHA injected to by improvement in the patients’ voice. Because of this limitation, we err on the side of under-correction for fear of the potential consequences of over-correction. We inform all patients of this limitation preoperatively and prepare them for the possibility of requiring a second injection at some point in the future. Removing the needle slowly from the vocal fold helps seal the injection site and limit implant extrusion.
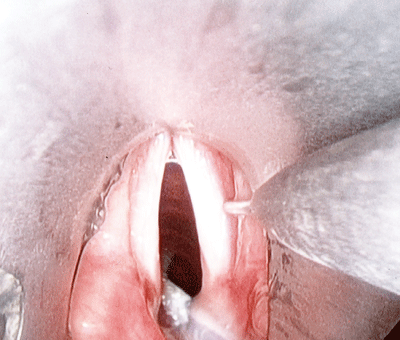
Figure 5.
Laryngeal injection needle delivering 0.2cc of implant just lateral to right thyroarytenoid muscle. Hunsacker jet ventilation needle can be seen below in the posterior glottis.

Fgure 6.
Black dots mark the site of injection augmentation. The injection is made lateral and deep in the TA muscle just anterior to the vocal process.